Galaxy Diagnostics Launches Nanotrap® Urine Test for Lyme Borreliosis
When we announced our partnership with Galaxy Diagnostics — a social venture and One Health company on a mission to build awareness and offer the best testing for flea and tick-borne infections — we knew they were poised to offer innovative technology in confirming the detection of Bartonella infection and Lyme Borrelia infection. We are excited to have them as a Founding Lab on our network because their innovation continues to drive advancements in the industry.
Groundbreaking, Revolutionary Testing
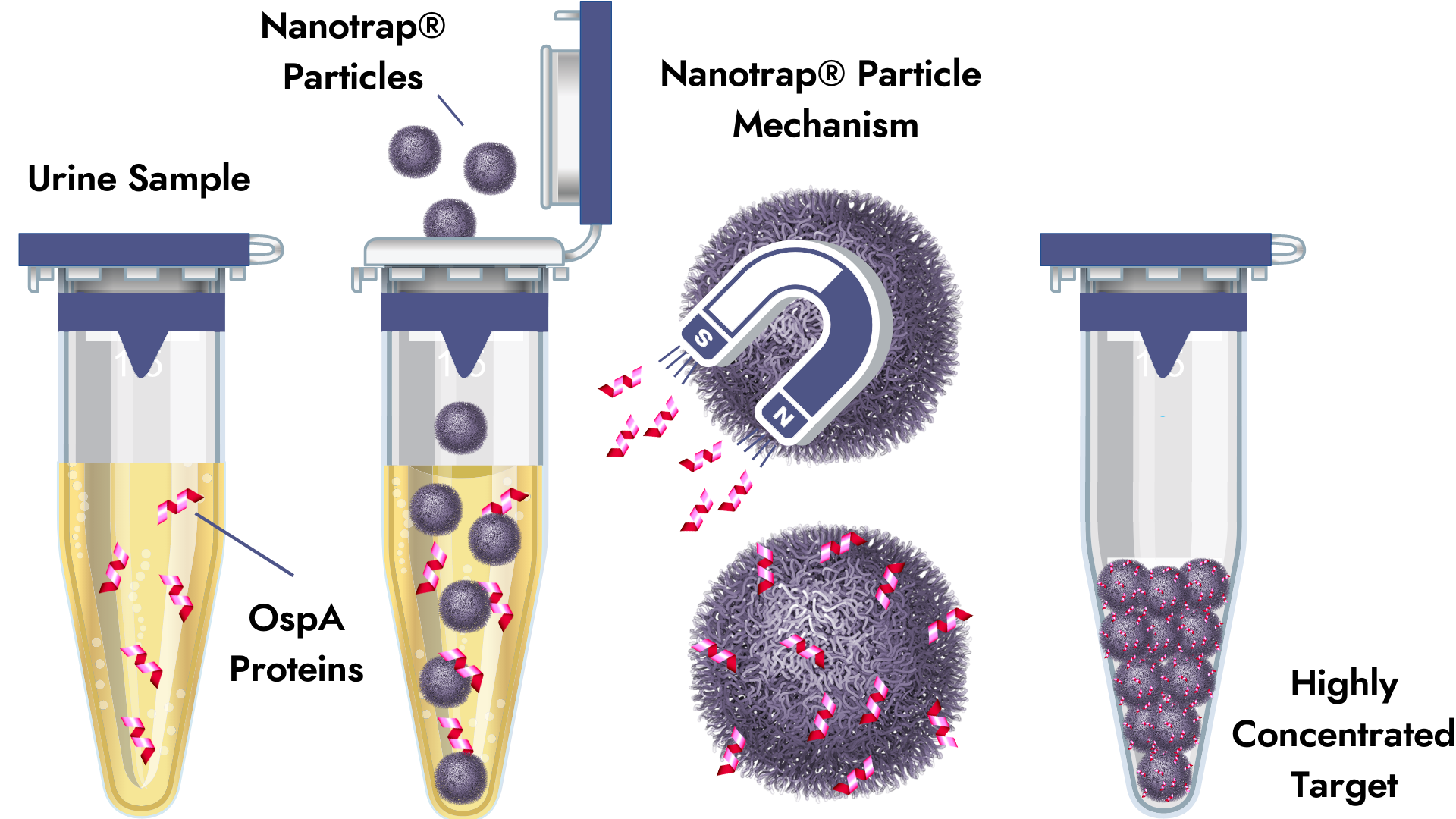
Their Nanotrap® Urine Test highlights the unique innovation Galaxy Diagnostics offers patients and physicians. The test is revolutionary in that it provides more sensitive direct detection for Borrelia burgdorferi infection at all stages of the disease. CDC-recommended Two-Tiered Testing (TTT) has long been the standard in detecting the Borrelia burgdorferi, but recent research suggests it likely misses early-stage infections. With Lyme disease, where early diagnosis and prompt treatment are critical, the Nanotrap® Urine Test identifies cases missed by TTT in both the earliest stages of infection (during the first four to six weeks) and later stages of the disease.
Additionally, the test reduces concern for false positive results by direct detection of Outer surface protein A (OspA) antigen, which prior research has shown is detectable in the urine of Lyme disease patients. Borrelia burgdorferi is known to aggregate in the bladder and is an important means of confirming infection in rodent models of Lyme Disease.
The Nanotrap® Urine Test offers the convenience of detection through urine samples, which is a big advantage for children, the elderly, and those who dislike needles. Urine also has fewer inhibitory substances than blood which may interfere with the detection of the target (protein or DNA).
Why OspA?
Outer Surface Protein A is a Lyme Borrelia-specific protein that is expressed on the outer surface of the bacterial membrane. As a tick adhesin, it sticks to the midgut of a tick and remains inside during feeding. Lyme Borrelia species differentially regulate OspA expression to help “sense” the environment around them, which makes its detection so important.
According to Dr. Jennifer Miller, Director of Research and Development and Assistant Laboratory Director at Galaxy Diagnostics, “Direct detection is like trying to detect a needle in a haystack.”
The ability of Nanotrap® particles to capture and concentrate small amounts of Borrelia antigen circumvents the limitations of both PCR and antibody testing. B. burgdorferi organisms and their surface proteins are present at very low concentrations in biological fluids, making direct detection a challenge, hence the “needle in the haystack” analogy. Due to specialized chemistry, Nanotrap® particles have a specific affinity for OspA and will selectively bind to or “capture” it.
“The CDC standard two-tier testing methodology and other antibody tests are indirect, as they measure antibody responses, which provide evidence of pathogen exposure but do not provide direct evidence of active infection,” Dr. Miller said. “With Nanotrap® testing, it’s a direct detection test, so it provides direct evidence of an active infection.”
It’s important to note, there is no test currently available that can detect every instance, symptom or circumstance of Lyme Borreliosis. Because of this, Dr. Miller recommends combining direct and indirect detection methods to minimize the possibility of misdiagnosis.
Galaxy Diagnostics continues to work with academic research partners to further delineate all the disease stages and presentations detectable with the Nanotrap® Urine Test.
We’re thrilled to be their partner and look forward to seeing the advancements and convenience they’ll offer patients and physicians, alike.
Before you go…
